Which Element Commonly Has Only a Proton as Its Nucleus
It has its nucleus and its single proton. And we know that the atomic number of nitrogen is 7 so the number of protons in nitrogen is 7.
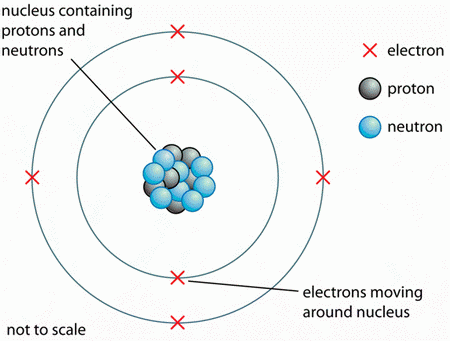
What Do All Atoms Of All Elements Have In Common Socratic
It has a single proton in its nucleus and no neutrons.

. Hydrogen is the only element in entire periodic table with only 1 proton in its nucleus. SolutionThe atomic number of iodine 53 tells us that a neutral iodine atom contains 53 protons in its nucleus and 53 electrons outside its nucleus. The element is Hydrogen.
A radioactive isotope of nitrogen has 9 neutrons. It is a plentiful non-metal whose color is yellow. Why nitrogen has 7 atomic number.
Hydrogen has only one proton atomic number 1. We must then look for an element that has an atomic number equal to 16. There are only certain combinations of neutrons and protons which forms stable nuclei.
The atomic number of a chemical element is the total number of protons that each atom of that element has. Hydrogen H is the one and only element in the periodic table containing one proton. Only one element has single protonproton is correct spelling.
The correct answer is d. Answer by studying proton collisions hope this. A chemical element of atomic number 16 is sulfur and its symbol is S.
For example the most common nuclide of the common chemical element lead Pb has 82 protons and 126 neutrons. As the atomic number of an atom denotes the number of proton as well as the number of electrons in it Hydrogen having the atomic number one and being the very first element in periodic table it has only one proton. A nitrogen atom has 7 protons and its most common isotope has 7 neutrons.
A singleelectron revolves around in s subshell of. The number of electrons in an atom is always equal to the number of protons.
The Structure Of The Atom Boundless Chemistry
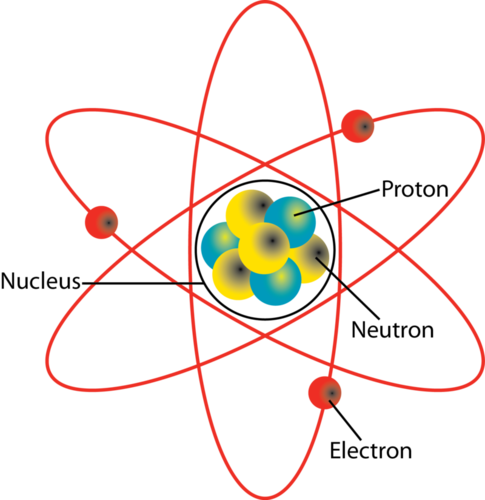
What Two Particles Are Found In The Nucleus Of An Atom Socratic

Comments
Post a Comment